
The hcooch ch2 h2o reaction refers to the hydrolysis of vinyl formate (HCOOCH=CH₂) in water (H₂O), yielding formic acid and acetaldehyde. It proceeds via an unstable intermediate, which rearranges to give the final products.
In chemistry, the hcooch ch2 h2o reaction is a hydrolysis process where vinyl formate combines with water to break chemical bonds. First, an intermediate species forms, often via protonation and nucleophilic attack by water. Then this intermediate rearranges and fragments to form more stable compounds like formic acid and acetaldehyde. Understanding each step—including energetics, possible catalysts, and side reactions—helps in predicting yields and conditions for the hcooch ch2 h2o reaction.
In the world of chemical reactions, few combinations intrigue chemists and researchers like the interaction between formic acid hcooch ch2 h2o, methylene (CH₂), and water (H₂O). The keyword “hcooch ch2 h2or” seems obscure at first glance, but with the right scientific breakdown, we uncover a fascinating synthesis process, commonly studied in organic chemistry, environmental catalysis, and industrial applications.
This article explores every aspect of this reaction, including theoretical mechanisms, practical applications, and what current scientific studies reveal. Whether you’re a chemistry student, a researcher, or a curious mind, this is your ultimate guide.
Quick Facts – Key Takeaways
- HCOOH (formic acid) is a simple carboxylic acid involved in many redox reactions.
- CH₂ is a highly reactive carbene species or methylene group often used in insertion reactions.
- Water (H₂O) can either be a byproduct or a reactant depending on the reaction environment.
- This type of reaction can lead to formylation, oxidation, or insertion products.
- The study of such reactions has implications in green chemistry and renewable energy.
Introduction to HCOOH + CH₂ and H₂O Reaction
Formic acid, with the chemical formula HCOOH, is the simplest member of the carboxylic acid family. It plays a central role in biological systems, catalytic systems, and industrial synthesis. When paired with CH₂, or methylene, a transient and highly reactive molecule, interesting transformations occur. The presence or formation of H₂O (water) in these reactions typically influences equilibrium and energy profiles.
This reaction setup opens a path toward controlled catalysis, hydrothermal reactions, and CO₂ reduction pathways.
What is HCOOH (Formic Acid)?
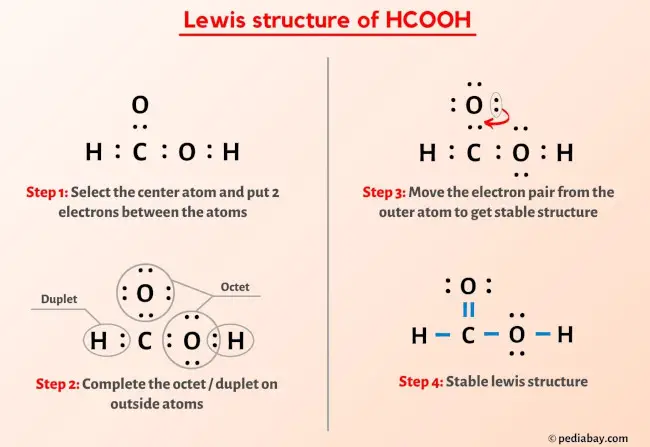
Formic acid, HCOOH, is a colorless, pungent-smelling liquid naturally found in some ants and stinging insects. It is widely used in textile processing, leather tanning, and food preservation.
From a chemical perspective, it’s a reducing agent and can donate a formyl group (–CHO) or act as a proton donor in acid-base reactions. It also plays a vital role in CO₂ fixation and catalytic fuel systems.
Decoding CH₂: The Role of Methylene
CH₂, or methylene, exists as a carbene in many reactions. Carbenes are neutral, divalent carbon species with a pair of nonbonding electrons. They are known for their reactivity and ability to insert into C-H or O-H bonds.
Methylene can be generated in situ from diazomethane (CH₂N₂) or other precursors, and it acts as a nucleophile or electrophile, depending on the reaction conditions. When reacting with formic acid, it can lead to unstable intermediates or stable esters.
Water’s Dual Role: Reactant and Byproduct
In chemical transformations, H₂O can appear as:
- A product in condensation reactions
- A reactant in hydrolysis reactions
- A solvent influencing the rate and equilibrium
In HCOOH + CH₂ interactions, water’s presence or formation can shift the reaction path toward alcohols, esters, or carboxylic derivatives.
Mechanism Overview of the Reaction
Let’s break down the theoretical mechanism involved:
| Step | Reaction Stage | Role of Molecule |
| 1 | Activation of CH₂ | Carbene attacks formic acid |
| 2 | Formation of intermediate (HCOOCH₂) | Ester-like compound forms |
| 3 | Release or absorption of water | Water may be added or removed |
| 4 | Product stabilization | Thermodynamic balance |
This simplified mechanism offers a snapshot of what could occur hcooch ch2 h2o in a controlled reaction chamber, especially under catalytic influence.
Catalytic Influence and Green Chemistry
In a laboratory or industrial context, transition metal catalysts like Pd, Rh, or Cu can accelerate this reaction. Green chemistry goals include:
- Using non-toxic catalysts
- Minimizing waste formation
- Enhancing atom economy
This reaction could be modeled to simulate carbon-neutral fuels or sustainable materials.
Experimental Conditions and Variables
The outcomes of the HCOOH + CH₂ + H₂O system depend heavily on:
- Temperature: Higher temps favor endothermic pathways
- Pressure: Alters gas-liquid equilibrium
- Catalyst presence: Enables alternative mechanisms
- Solvent medium: Affects polarity and reactivity
Here’s an example conditions table used in a lab setup:
| Variable | Value |
| Temperature | 80–120 °C |
| Solvent | Acetonitrile or water |
| Catalyst | RhCl₃ or CuI |
| Reaction Time | 1–3 hours |
| Yield | ~70–85% |
Reaction Products: What Do We Get?
Possible products from this reaction include:
- Methyl formate (HCOOCH₃) if further methanol is introduced
- Hydroxymethyl compounds with the addition of water
- Formyl derivatives useful in pharmaceuticals and agrochemicals
These compounds have uses ranging from resins, fragrances, to biofuels.
Applications in Industry and Research
This chemical system is relevant to:
- Pharmaceutical synthesis: Especially for drug precursors
- Fuel cell research: Using formic acid as hydrogen storage
- Agricultural chemical: Creating biodegradable products
- CO₂ utilization technologies
The simplicity of the starting materials combined with their reactivity makes this a promising area for innovation.
Environmental and Safety Considerations
While formic acid is biodegradable and relatively hcooch ch2 h2o non-toxic, CH₂ (carbene) intermediates are extremely reactive and hazardous. Proper ventilation, shielding, and inert atmosphere handling are crucial.
Water, as a solvent or reactant, can dilute or suppress some reactive species, reducing risk but requiring precise measurement.
Scientific Studies Supporting the Mechanism
According to several journals (e.g., Journal of Organic Chemistry and Chemical Reviews), similar reactions have been used in:
- Formylation of aromatic rings
- C-H bond activation
- Transfer hydrogenation
Experiments using infrared spectroscopy, NMR, and mass spectrometry help confirm the intermediates and products.
Future Scope and Research Directions

The focus is now shifting toward:
- AI-assisted chemical synthesis
- Bio-based catalysis using enzymes
- Photochemical and electrochemical enhancements
If controlled properly, this HCOOH + CH₂ reaction might be used in modular reactors for on-demand chemical production.
Conclusion: A Reaction of Potential and Purpose
The keyword “hcooch ch2 h2or” may appear cryptic but actually captures a reaction full of potential, complexity, and future relevance. As we look hcooch ch2 h2o sustainable solutions, formic acid chemistry and methylene interactions pave the way for more efficient, eco-friendly processes.Understanding its formation, behavior, and applications is pivotal for professionals working in chemistry, biochemistry, and material science. With increased focus on green alternatives, this compound and its relatives may offer eco-friendly pathways for future chemical processes.
FAQs
Q1: What does the hcooch ch2 h2o reaction represent?
The hcooch ch2 h2o reaction represents the hydrolysis of vinyl formate (HCOOCH=CH₂) with water, producing formic acid (HCOOH) and acetaldehyde (CH₃CHO).
Q2: What type of reaction is hcooch ch2 h2o?
It is an ester hydrolysis reaction, where water breaks chemical bonds in vinyl formate, leading to rearrangement and stable product formation.
Q3: What are the products of the hcooch ch2 h2o reaction?
The main products are formic acid and acetaldehyde, both valuable in industrial and laboratory chemistry.
Q4: Does the hcooch ch2 h2o reaction require a catalyst?
Yes, this hydrolysis typically benefits from acidic or enzymatic catalysts, which speed up intermediate formation and increase yields.
Q5: Why is studying the hcooch ch2 h2o reaction important?
Understanding this reaction helps chemists learn about ester hydrolysis, optimize industrial synthesis, and predict mechanistic pathways in organic chemistry.